Snaccine 2025
a phage-based edible vaccine platform
NitroBLAST 2024
Laying the foundation for nitrogen fixation
Spyke 2022
Combating drink spiking with biotechnology
AptaVita 2021
Empowering accessible diagnostics to expose hidden hunger
PHOCUS 2020
Targeting locust swarms with precise and sustainable biocontrol
Sci-Phi 2019
Expanding the bacterial toolbox for versatile synthetic biology
ADOPE 2018
Racing to keep sports clean through gene doping detection
Case13a 2017
Fighting antibiotic resistance through rapid on-site diagnostics
Opticoli 2016
Illuminating microscopy through biological optics and lasers
Biolink 2015
Structuring biofilms through precision 3D printing
Electrace 2014
Electrifying biosensors through bacterial electron transport
Peptidor 2013
Targeting MRSA through smart bacterial sentinels
Snifferomyces 2012
Engineering yeast cells to sniff out disease markers
StickE. Coli 2011
Making bacteria stick through engineered mussel proteins
Alkanivore 2010
Engineering bacteria to clean up oil spills and save our oceans
Relay Race 2009
Engineering bacteria to relay messages across colonies with precision and control

What are snaccines?
With snaccines, the vaccine factory is in your gut. Phages, viruses for bacteria, are the star of the show. Our edited phages make bacteria in the gut produce the mRNA vaccine. This brings many advantages over both traditional vaccines and mRNA vaccines. DNA phages can be kept at much higher temperatures (room temperature) than mRNA vaccines are (-80°C). They don't require nearly as much chemical processing. Like mRNA vaccines, it takes hours, not years, to develop a vaccine. And a snaccine doesn't require an injection, but can be taken orally. This makes our platform ideal for fighting bird flu, neglected tropical diseases or the next pandemic.

What is iGEM?
The International Genetically Engineered Machine (iGEM) competition is a prestigious global platform in synthetic biology. Since its inception in 2004, iGEM has grown to involve hundreds of teams from universities and research institutions worldwide. It brings together students from diverse academic disciplines to create innovative solutions to pressing global challenges.
Participants tackle real-world issues like bioremediation, disease treatment, food security, and environmental sustainability. By combining experimental biology with computational tools such as modeling and software development, iGEM projects uniquely blend life sciences, engineering, and social impact.
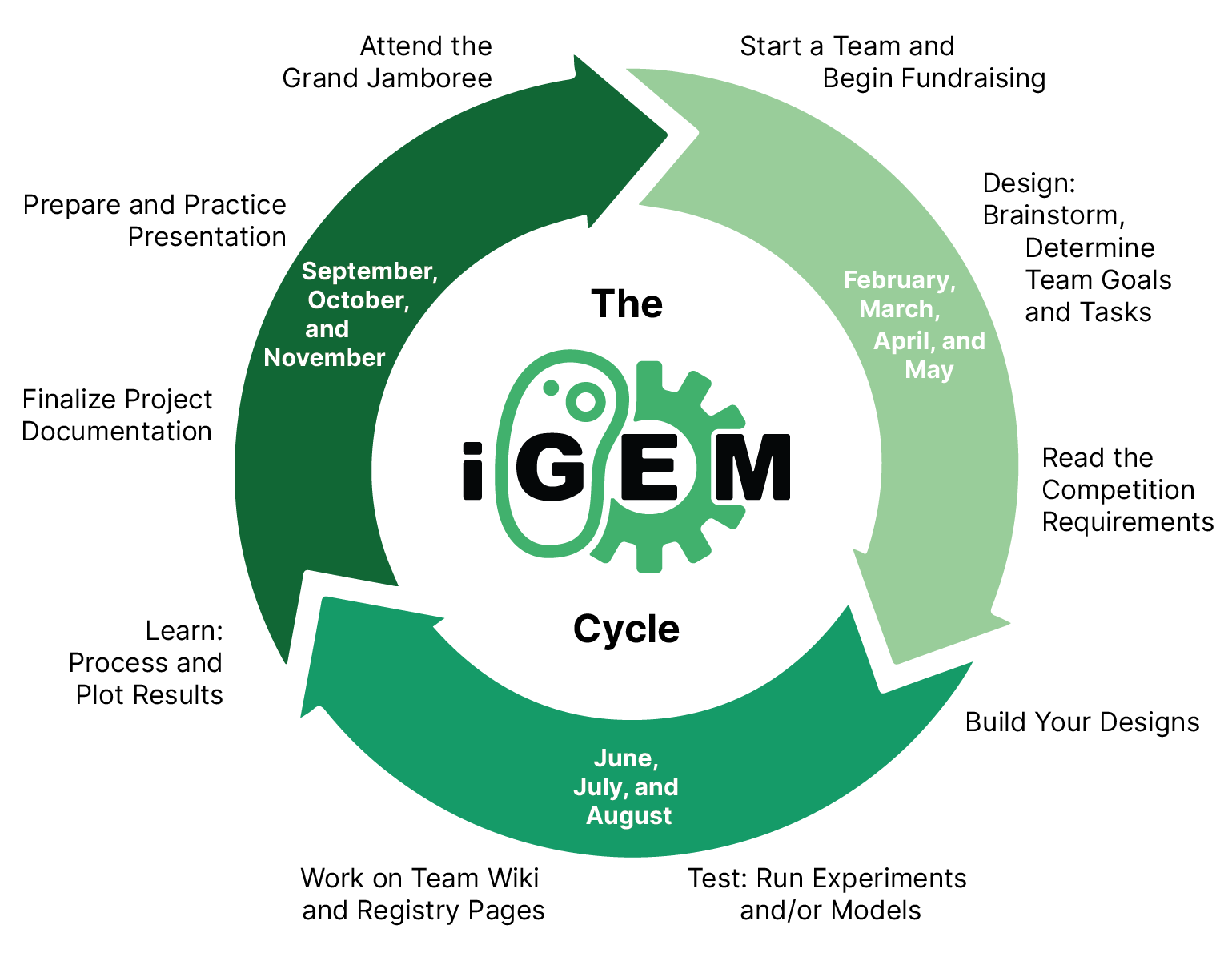
How Does iGEM Work?
The competition follows a structured timeline:
- Team Formation & Research (Winter/Spring): Teams come together, brainstorm, and plan their project goals.
- Design, Build, Test (Summer): Over the summer, teams develop and refine their synthetic biology systems.
- Giant Jamboree (Fall): Held in Paris, France, this event is the culmination of iGEM. Teams present their work to a global audience, compete for awards, and receive feedback from leading experts in the field.

Why Join iGEM at TU Delft?
For 2026, we will be seeking passionate, talented students from across TU Delft’s faculties to join our team. Whether your background is in biology, engineering, computer science, design, or social sciences, your skills can help shape groundbreaking solutions in synthetic biology. By joining the TU Delft iGEM team, you will:
- Gain hands-on experience in cutting-edge scientific research.
- Work in an interdisciplinary, collaborative environment.
- Contribute to projects that address global challenges and make a meaningful societal impact.
Beyond lab work, our iGEM team offers opportunities to:
- Engage in project design and innovation.
- Develop computational tools and software.
- Lead public outreach efforts, making science accessible to broader audiences.




